1.Zhu, L, et al. (2020). "Bioanalytical Challenges in Support of Complex Modalities of Antibody-Based Therapeutics." AAPS J 22(6): 130.
2.Ma, M., et al. (2019). "Bioanalytical challenges and unique considerations to support pharmacokinetic characterization of bispecific biotherapeutics." Bioanalysis 11(5): 427-435.
3.Seimetz D. Novel monoclonal antibodies for cancer treatment: the trifunctional antibody catumaxomab (removab). J. Cancer 2, 309–316 (2011).
4.Mullard A. Bispecific antibody pipeline moves beyond oncology. Nat. Rev. Drug Discov. 16(11), 666–668 (2017).
5.Diao L, Meibohm B. Tools for predicting the PK/PD of therapeutic proteins. Expert Opin. Drug Metab. Toxicol. 11(7), 1115–1125 (2015).
6.Trivedi A, et al. Clinical pharmacology and translational aspects of bispecific antibodies. Clin. Transl. Sci. 10(3), 147–162 (2017).
7.Ezan E, et al. Assessment of the metabolism of therapeutic proteins and antibodies. Expert Opin. Drug Metab. Toxicol.10(8), 1079–1091 (2014).
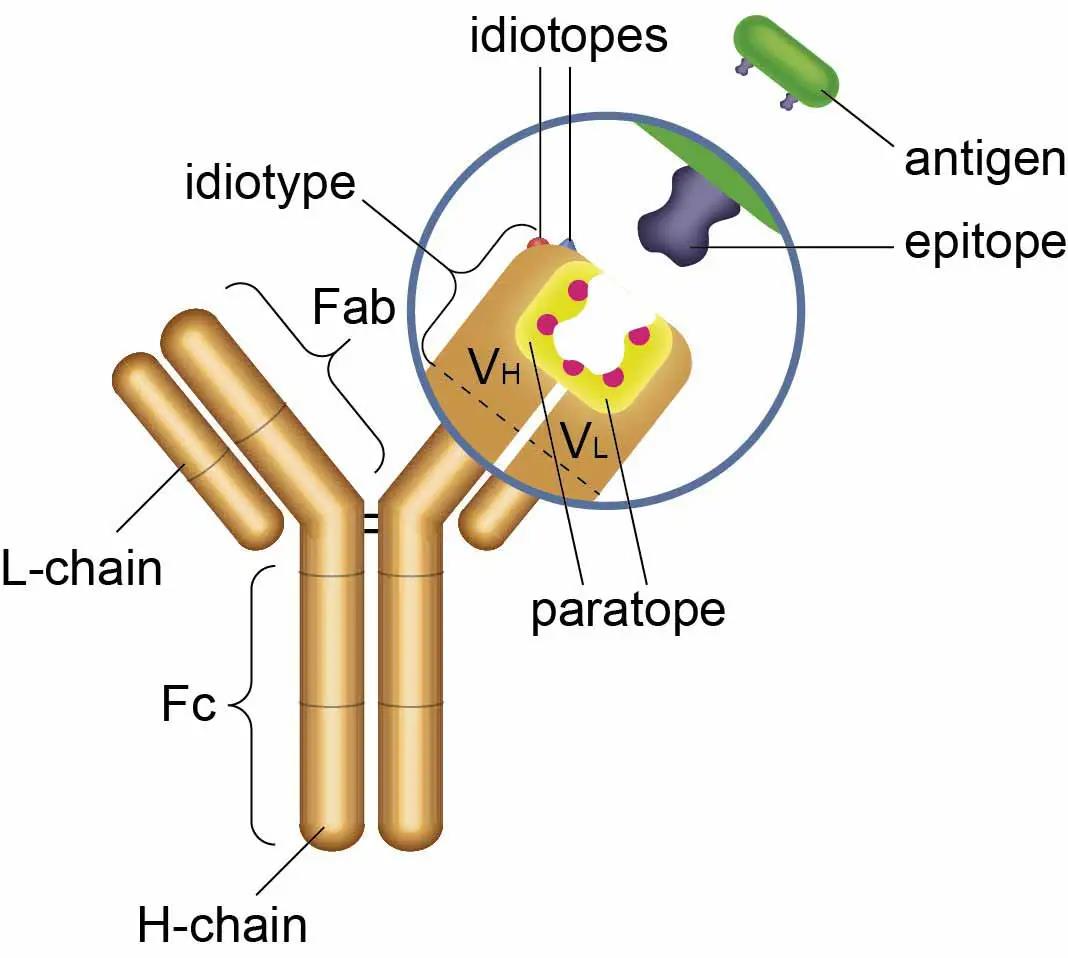
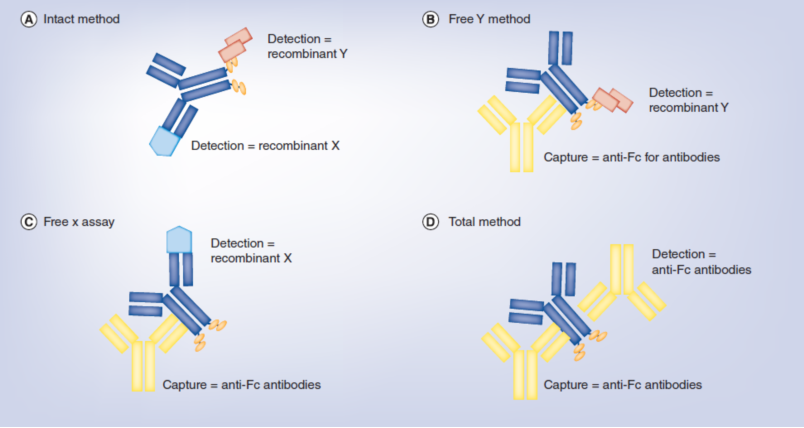
8.Fischer SK, et al. The assay design used for measurement of therapeutic antibody concentrations can affect pharmacokinetic parameters. Case studies. MAbs 4(5), 623–631 (2012).
9.Ruf P, et al. Pharmacokinetics, immunogenicity and bioactivity of the therapeutic antibody catumaxomab intraperitoneally administered to cancer patients. Br. J. Clin. Pharmacol. 69(6), 617–625 (2010).
10.Samineni D, et al. Impact of shed/soluble targets on the PK/PD of approved therapeutic monoclonal antibodies. Exp. Rev. Clin. Pharm. 9(12), 1557–1569 (2016).
11.Villegas VM, et al. Current advances in the treatment of neovascular age-related macular degeneration. Expert Opin. Drug Deliv. 14(2), 273–282 (2017).
12.Ruppel J, et al. Preexisting antibodies to an F(ab’)2 antibody therapeutic and novel method for immunogenicity assessment. J. Immunol. Res. 2016, 1–8 (2016).
13.Fan X, et al. Lens glutathione homeostasis: discrepancies and gaps in knowledge standing in the way of novel therapeutic approaches. Exp. Eye Res. 156, 103–111 (2017).
14.Kang L, et al. LC-MS bioanalysis of intact proteins and peptides. Biomed Chromatogr. 2020;34(1):e4633. https://doi.org/10.1002/bmc.4633.
15.Chen, J, et al. "Capillary nano-immunoassays: advancing quantitative proteomics analysis, biomarker assessment, and molecular diagnostics." Journal of translational medicine 13: 182. (2015)
16.Murphy RE, et al. Combined use of immunoassay and twodimensional liquid chromatography mass spectrometry for the detection and identification of metabolites from biotherapeutic pharmacokinetic samples. J Pharmaceut Biomed.2010;53(3):221–7. https://doi.org/10.1016/j.jpba.2010.04.028.
17.He JT, et al. High resolution accurate-mass mass spectrometry enabling in-depth characterization of in vivo biotransformations for intact antibody-drug conjugates. Anal Chem. 2017;89(10):5476–83.https://doi.org/10.1021/acs.analchem.7b00408.
18.Jian WY, et al. A workflow for absolute quantitation of large therapeutic proteins in biological samples at intact level using LC-HRMS. Bioanalysis.2016;8(16):1679–91. https://doi.org/10.4155/bio-2016-0096.
19.Lanshoeft C, et al. Generic hybrid ligand binding assay liquid chromatography high resolution mass spectrometry based workflow for multiplexed human immunoglobulin G1 quantification at the intact protein level: application to preclinical pharmacokinetic studies. Anal Chem. 2017;89(4):2628–35. https://doi.org/10.1021/acs.analchem.6b04997.
20.Jin W, et al. LC-HRMS quantitation of intact antibody drug conjugate trastuzumab emtansine from rat plasma. Bioanalysis. 2018;10(11):851–62. https://doi.org/10.4155/bio-2018-0003.
21.Zhang LY, et al. Top-down LC-MS quantitation of intact denatured and native monoclonal antibodies in biological samples. Bioanalysis. 2018;10(13):1039–54. https://doi.org/10.4155/bio-2017-0282.
22.Li Y, et al. An efficient and quantitative assay for epitope-tagged therapeutic protein development with a capillary western system. Bioanalysis. 2019;11(6):471–84. https://doi.org/10.4155/bio-2018-0248.
23.Kodani M, et al. An automated immunoblot method for detection of IgG antibodies to hepatitis C virus: a potential supplemental antibody confirmatory assay. J Clin Microbiol. 2019;57(3). https://doi.org/10.1128/JCM.01567-18.
关于JDB电子医药 临床研究服务:
JDB电子医药拥有一支规模庞大、专业成熟的临床研究队伍,可提供包括医学、项目管理、监查、稽查、数据管理和统计分析、生物样本检测在内的临床试验全流程解决方案。截至2020年,JDB电子医药服务的客户超1000家,完成800多项临床试验项目,助力客户获得新药证书60多项、生产批件超过80项。拥有丰富的临床试验服务经验,服务项目涵盖临床研究各个领域,在肿瘤、肝病、消化等创新药领域拥有独特的临床服务体系。
JDB电子医药在全国设有40多个临床监查网点,与全国近600个临床试验机构展开合作,并运用ORACLE OC/RDC及CTMS系统,控制临床数据采集的及时性、管理临床试验过程的规范性。